Prepare a table featuring more examples of isotopes, isobars and isotones.
Isotopes – These are the atoms of the same element having the same number of protons but different number of neutrons or we could also say that these are the chemical species having the same atomic number but different mass numbers.
Table featuring examples of isotopes : -
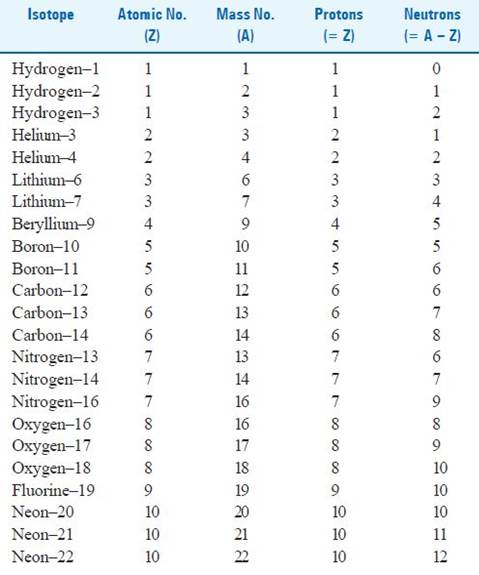
Isobars – The atoms which have the same mass number but different atomic number are called isobars.
Table featuring examples of isobars

Isotones - Two atoms are called isotones if they have the same number of neutrons N, but different number of protons Z.
Table featuring examples of isotones

5