Draw a sketch of Bohr's model of an atom with three shells.
A sketch of Bohr's model of an atom with three electron shells is shown below:
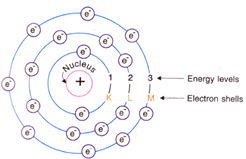
According to Bohr’s model of an atom:
(i) Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom.
(ii) While revolving in discrete orbits the electrons do not radiate energy.
These orbits are called energy levels. Energy levels in an atom are shown by circles.
These orbits are represented by the letters K,L,M,N,… or the numbers, n=1,2,3,4,….
Further, the distribution of electrons into different orbits of an atom was suggested by Bohr and Bury. The following rules are the rules followed for writing the number of electrons in different energy levels or shells: (i) The maximum number of electrons present in a shell is given by the formula 2n2, where ‘n’ is the orbit number or energy level index, 1,2,3,…. Hence the maximum number of electrons in different shells can be written as : first orbit or K-shell will be = 2 × 12 = 2, second orbit or L-shell will be = 2 × 22 = 8, third orbit or M-shell will be = 2 × 32 = 18, fourth orbit or N-shell will be = 2 × 42= 32, and so on. The maximum number of electrons that can be accommodated in the outermost orbit is 8. Electrons are not accommodated in a given shell, unless the inner shells are filled. That is, the shells are filled in a stepwise manner.