Element ‘M’ forms a chloride with the formula MCl2 which is sold with high melting point. To which group of the Periodic table does the element ‘M’ belong?
Element M should belong to group 2 of the periodic table.
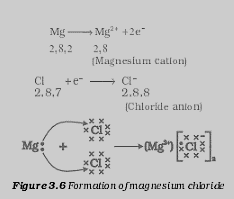
Reason: Since the chloride formula formed by the metal is of the form MCl2, the valency of the metal is +2. Also, it is high melting solid so it has to be an ionic compound and so metal should be definitely from 1st or 2nd group. But since metals of group 1 can show only +1 valency, the metal cannot be from group 1 then it is definitely from group 2. Ex: Ca, Mg etc. are 2nd group metals and satisfy this situation.
1