With the help of a suitable example, explain how ionic compounds are formed State any three general properties of ionic compounds.
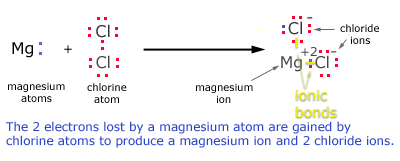
Let us take the example of an ionic compound MgCl2.it consists of cation ![]() . Mg in its pure form consists two extra electrons on of the other hand chlorine is sort of one electron to complete its octet. Hence one magnesium and two chlorine come together to transfer electrons between them and form the ionic compound. Formation of all ionic compounds take place in similar fashion.
. Mg in its pure form consists two extra electrons on of the other hand chlorine is sort of one electron to complete its octet. Hence one magnesium and two chlorine come together to transfer electrons between them and form the ionic compound. Formation of all ionic compounds take place in similar fashion.
Three general properties of ionic compounds are as follows:
1) Soluble in polar solvents and insoluble in organic solvents.
2) Good conductor in molten form but non-conductor in solid forms.
3) generally found in crystalline solid forms due to strong force of interaction.