Four metal A, B, C and D are added to the following aqueous solutions one by one. The observations made are tabulated below:
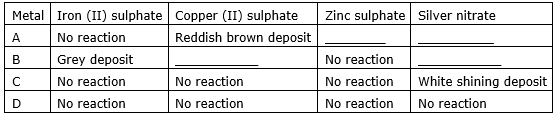
Answer the following questions based on the above observations:
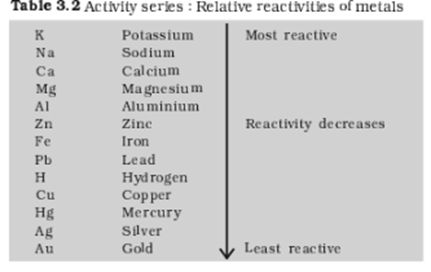
(a) Which is the most active metal and why?
(b) What would be observed if B is added to a solution of copper (II) sulphate and why?
(c) Arrange the metal A, B, C and D in order of increasing reactivity.
(d) The container of which metal can be used to store both zinc sulphate solution and silver nitrate solution?
(e) Which of the above solutions can be easily stored in a container made up of any of these metals?
(a) The most reactive metal is metal B because it can react with iron and all the metals which come below in reactive series than iron. Hence in the table, it can react with all metal sulphates except zinc sulphate.
(b) A reddish brown deposit of copper will be formed as B will react with all other metal sulphates except zinc.
(c) B>A>C>D
reason: The more reactive metal sulphates with which it can react the more reactive it is.
(d) Container of metal D will be appropriate because it doesn’t react with any of the two.
(e) Zinc sulphate can be stored in any container made from these metals because it doesn’t react with any of them.