A vegetable oil contains two double bonds in its molecule. How many moles of hydrogen gas are required for complete hydrogenation of 1 mole of oil?
Consider the following reaction:
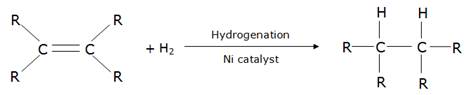
Note that one double bond is hydrogenated using 1 mole of H2.
∴ if there are 2 double bonds in the vegetable oil, then 2 moles of H2 will be required for complete hydrogenation of 1 mole of the oil.
5