Why are carboxylic acids called weak acids? Name the alcohol which produces methanoic acid on oxidation.
The strength of acid is determined by its ability to produce H+ in solutions. Unlike mineral acids, the carboxylic acids (of the form RCOOH) do not completely ionise. They partially ionise to give H+ and RCOO-. Therefore they are called weak acids.
Methanol on oxidation in the presence of a strong oxidising agent like alkaline KMnO4 produces methanoic acid.
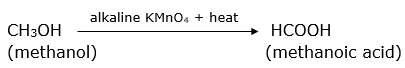
17