Complete the following reactions:
(i) CH3COOH + NaOH →
(ii) ![]()
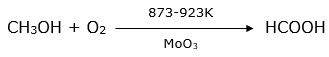
(iii) CH3OH + O2![]()
(iv) CH2 = CH2 + Br →
(v) CH3COOC2H5 + NaOH →
(vi) CH2 = CH2 + H2O ![]()
(i) This is an example of neutralization reaction
CH3COOH + NaOH → CH3COONa + H2O
(ii) 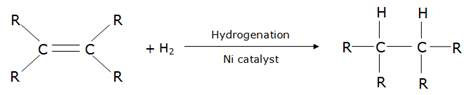
(iii) Alcohols will get oxidised to acids in the presence of MoO3 which is an oxidising agent. In this case, methanol (CH3OH) gets oxidised to methanoic acid (HCOOH).
(iv) CH2 = CH2 + Br → CH2Br—CH2Br
This is an example of addition reaction.
(v) CH3COOC2H5 + NaOH → CH3COOH + C2H5OH
This is an example of saponification reaction and is used in the preparation of soaps.
(vi) ![]()
This is an addition reaction, where H+ is added to one of the carbon and OH- is added to the other carbon.
18