(a) Identify the compounds ‘A’, ‘B’ and ‘C’ in the following reactions:
(i) C2H5OH + CH3CH2COOH ![]() ‘A’ + H2O
‘A’ + H2O
(ii) CH3COOC2H5 + NaOH →‘B’ + C2H5OH
(iii) CH3COONa + NaOH(CaO) ![]() ‘C’ + Na2CO3
‘C’ + Na2CO3
(b) A cyclic compound ‘X’ has molecular formula C6H6. It is unsaturated and burns with a sooty flame. Identify ‘X’ and write its structural formula. Will it decolourise bromine water or not and why?
(a) (i) A – CH3CH2COOC2H5
(ii) B – CH3COOH
(iii) C – CH4
(b) The compound X is benzene (C6H6). The structural formula is
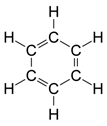
Generally, bromine water gets decolourised when it is added to an unsaturated compound. But the double bonds in the benzene are delocalised which increases its stability or decreases its reactivity. Benzene will not undergo addition reaction with bromine water.
Therefore, benzene does not decolourise bromine water.