(a) A compound ‘X’ is C2H4. Draw its electron dot structure. Will it dissolve in water or not? Will it conduct electricity in aqueous solution? Will it have high melting or low melting point?
(b) A compound ‘X’ has molecular formula C4H10. It undergoes substitution reaction readily than addition reaction. It burns with blue flame. It is present in LPG. Identify ‘X’ and give the balanced equation for its combustion and substitution reaction with Cl2 in presence of sunlight.
(c) Why are vegetable oils healthy as compared to vegetable ghee? How are vegetable oils converted into vegetable ghee? Name the process.
(a) The electron dot structure of ethene is
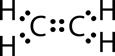
Ethene is a non-polar molecule and will not dissolve in water (polar solvent). Since it does not produce ions in water, ethene does not conduct electricity in aqueous solutions. Ethene is a covalent molecule and has low melting point.
(b) The compound X is butane.
Balanced equation for combustion of butane:
2C4H10 + 13O2→ 8CO2 + 10H2O
Substitution reaction with Cl2 in the presence of sunlight:
C4H10 + Cl2→ C4H9Cl + HCl
C4H9Cl + Cl2→ C4H8Cl2 + HCl
The substitution reaction continues until all H is replaced by Cl.
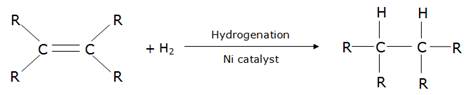
(c) Vegetable oils contain unsaturated carbon chains whereas vegetable ghee contains saturated carbon chains which are said to be harmful to health.
Vegetable oils are converted into vegetable ghee by the process of hydrogenation in the presence of a catalyst like nickel.