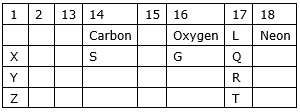
(a) Which is the most reactive metal?
(b) Which is the most reactive non-metal?
(c) Name the family of L, Q, R, T
(d) Name one element from each of groups 2, 13 and 15.
(a) Most reactive metal is Z because size of atom increases down a group and effective nuclear charge decreases. The attraction of nucleus on valence electrons also decreases. So, the tendency of losing electrons increases. Hence reactivity increases.
(b) We know that electronegativity of non-metals increases from left to right in a period due to the increment of effective nuclear charge and decreases down a group due to the increment of size of atom. So, the most reactive non-metal is L.
(c) L, Q, R, T are the elements of the halogen family.
(d) Group 2- Magnesium (Mg)
Group 13- Boron (B)
Group 15- Nitrogen (N)