An element belongs to 4th period and group 17 of the periodic table. Find out:
(a) the number of valence electrons.
(b) is it a metal or non-metal.
(c) the name of the element.
(d) formula of its compound with hydrogen.
(e) electron dot structure of this element with calcium.
(a) We know that every element of group 17 has 7 valence electrons. So this element also has 7 valence electrons.
(b) It is non-metal because it can only gain electron.
(c) Its name is Bromine (Br).
(d) The formula of its compound with hydrogen is HBr.
(e) 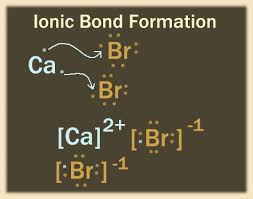
22