The positions of three elements A, B and C in the periodic table are indicated below:
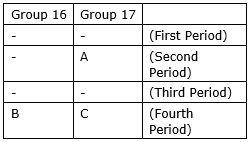
(a) State whether element C would be a metal or a non-metal. Why?
(b) Which is the more active element, A or C? Why?
(c) Which type of ion (cation or anion) will be formed by the element C? Why?
(a) Element C is non-metal because it belongs to 17 group and it contains 7 valence electrons so it can gain only one electron. So it is non-metal.
(b) In non-metals, electronegativity increases from left to right in a period due to increase in effective nuclear charge and decreases from top to bottom in a group due to increment in size. So A is the most active element.
(c) Since C is non-metal and it has 7 valence electrons. So it can gain only one electron and anion will be formed.