In the following table, six elements A, B, C, D, E and F (here letters are not the usual symbols of the elements) of the modern Periodic Table with atomic numbers 3 to 18 are given:
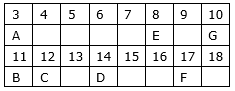
(a) Which of these is (i) a noble gas, (ii) a halogen?
(b) If B combines with F, what would be the formula of the compound formed?
(c) Write the electronic configurations of C and E.
(a) (i) G with atomic number 10 is a noble gas because it has complete octet.
(ii) F with atomic number 17 is a halogen.
(b) B with atomic number 11 has 1 valence electron and F with atomic number 17 has 7 valence electrons so the formula of the compound formed would be BF.
(c) Electronic configuration of C and E are
E8- 2, 6
C12- 2, 8, 2
27