In the following table, are given eight elements A, B, C, D, E, F, G and H (here letters are not the usual symbols of the elements) of the Modern Periodic Table with the atomic numbers of the elements in parenthesis.
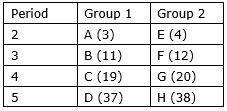
(i) What is the electronic configuration of F?
(ii) What is the number of valence electrons in the atom of F?
(iii) What is the number of shells in the atom of F?
(iv) Write the size of the atoms of E, F, G and H in decreasing order.
(v) State whether F is a metal or a non-metal.
(vi) Out of the three elements B, E and F, which one has the biggest atomic size?
(i) Electronic configuration of F is
F12- 2, 8, 2
(ii) There are 2 valence electrons in the atom of F.
Explanation- Valence electrons are those electrons which present in the last shell.
(iii) There are 3 shells in the atom of F.
(iv) We know that atomic size increases from top to bottom in a group due to increment in shells. So the order will be H>G>F>E.
(v) Since F has 2 valence electrons so it will donate these two electrons to acquire noble gas electronic configuration. Hence it is a metal.
(vi) We know that atomic size increases from top to bottom in a group and decreases from left to right in a period. So B has the bigger atomic size.