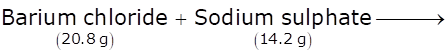In the chemical reaction given below, find the amount of barium sulphate formed. Also write the law involved
![]()
Let the amount of barium sulphate is “x”g
The given reaction is:
![]()
According to law of conservation of mass,
Amount of reactants = Amount of products formed
Hence, 20.8g + 14.2g = x + 11.7g
⇒ 35g = x + 11.7g
⇒ x = 23.5g
Thus, the amount of barium sulphate is 23.5g.
The law involved is Law of conservation of mass which states that
the sum of the masses of reactants and products remain unchanged.
17