Jyoti buys a gold jewellery which has 90% gold and the rest copper. She is given a bill which charges amount equivalent to 100% of gold. She argues with seller about the discrepancy, who settles the bill accordingly.
(i) What is the ratio of gold and copper in the jewellery?
(ii) How many atoms of gold are present in 1g of it?
(iii) What are the values displayed by Jyoti?
(i) The ratio of gold and copper in the jewellery is 90:10
(ii) Mass of gold in 1g jewellery = 90% × 1g
= 0.9g
Atomic mass of gold = 197g/mol
197 g = 6.022 × 1023 atoms
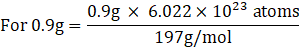
⇒ 2.72 × 1021 atoms
Thus, the number of atoms present in 1g of gold is 2.72 × 1021 atoms.
(iii) Jyoti is aware, concern and a knowledgeable person.
33