Four students A, B, C and D verified the law of conservation of mass by performing chemical reaction between barium chloride and sodium sulphate. All of them took 107.2 g barium chloride solution and 116.1g of sodium sulphate solution and mixed them in the beaker of mass 150 g. They reported their results as follows:
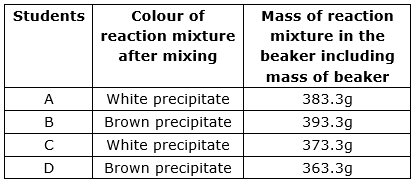
The correct observation is that of student.
Mass of barium sulphate =107.2 g
Mass of barium chloride = 116.1g
Mass of beaker = 150 g
Total mass of reaction mixture in the beaker including mass of
Beaker = 107.2g + 116.1g + 150g = 373.3g
4