During an experiment hydrogen (H2) and oxygen (O2) gases reacted in an electric are to produce water as
follows: 2H + O2![]() 2H2O
2H2O
The experiment is repeated three times and data tabulated as shown below:
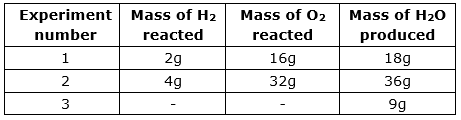
During 3rd experiment the researcher forgot to list masses of H2 and O2 used. So, if the law of constant proportion is correct then find mass of O2 used during 3rd experiment.
In experiment (i), the ratio is 2:16, i.e., 1:8
In experiment (ii), the ratio is 4:32, i.e., 1:8
According to the law of constant proportions, the ratio of third experiment should be the same (1:8)
Therefore, the mass of O2 used during 3rd experiment is 8g.
6