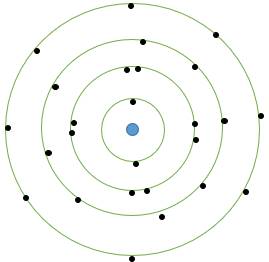
Draw a sketch of Bohr’s model of an atom with four shells.
The Bohr’s model of atom consisted a positively charged nucleus in the center of the atom and electron revolving in the discrete orbits or shells which are concentric and have specific number of electrons in it.
The first shell or orbit nearest to the nucleus was numbered as 1 and named K similarly the rest of shells were named and numbered.
Like n = 1, 2, 3…… ; K, L, M, N,……..
Where n is the shell number.
For Bohr’s model with 4 shells, the electrons no. are as follows:
First shell: 2 electrons
Second shell: 8 electrons
Third shell: 18 electrons
Fourth shell: 18 electrons
But maximum electrons in any shell can only be 8 due to octet rule.
7