The number of neutrons and protons present in the nuclei of two atomic species A and B are given below.
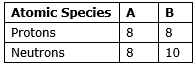
(i) Write the mass numbers of A and B.
(ii) What is the relation between two species?
(iii) Write the electronic configuration of atoms A and B.
For atomic species A:
Protons = 8
Neutrons = 8
For atomic species B:
Protons = 8
Neutrons = 10
(i) Mass number of A = 8 + 8 = 16
Mass number of B = 8 + 10 = 18
(ii) Both the species are isotopes of an element because they have the same atomic number or the number of protons.
(iii) Electronic configuration of A = 2, 6
Electronic configuration of B = 2, 6
23