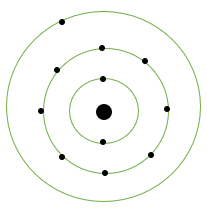
If K and L-shells of an atom are full and in M-shell, there is only one electron, then what would be the total number of electrons in the atom? Name the element. Write the symbol and draw the Bohr model.
If K and L shells of an atom are full and only one electron is present in the M shell, then the total number of the electrons in the atom would be 11 because the maximum number of electrons that can occupy K and L shells are 2 and 8 respectively. As only 1 electron is present in the M shell the total comes out to be 11.
Corresponding to 11 electrons there will be 11 protons in the atom, so the atomic number of the element will be 11. The element with atomic number 11 is Sodium which has the symbol Na.
26