(i) Draw the atomic structures of the following elements: Magnesium, silicon, sulphur.
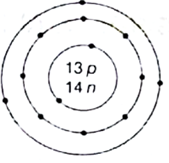
(ii) What is the atomic number and mass number of the element whose atomic structure is shown in below?
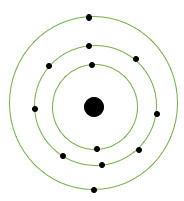
(i) Magnesium: Symbol = Mg
Atomic number = 12
Protons = 12
Electrons = 12
Electronic configuration = 2, 8, 2
Atomic structure:
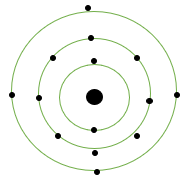
Silicon: Symbol = Si
Atomic number = 14
Protons = 14
Electrons = 14
Electronic configuration = 2, 8, 4
Atomic structure:
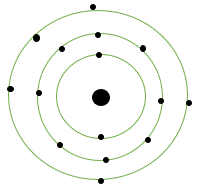
Sulphur: Symbol = S
Atomic number = 16
Protons = 16
Electrons = 16
Electronic configuration = 2, 8, 6
Atomic structure:
(ii)
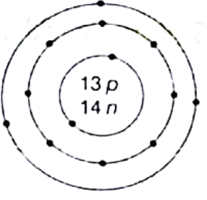
Atomic number that can be determined from the atomic structure is 13
Mass number is 13 + 14 = 27
33