The following diagram depicts Rutherford’s experiment.
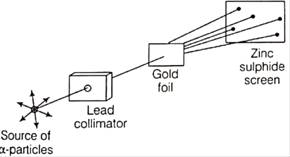
Why was zinc sulphide screen in used in the experiment?
Options || A. To block α-particles from going straight.
B. To detect deflection of α-particles.
C. To further deflect α-particles as the gold foil did.
D. To absorb α-particles and utilise it again.
Zinc sulphide screen was placed behind the gold foil to detect the deflection of the alpha particles from the gold foil. Every time any alpha particle hits the Zinc sulphide screen it shows fluorescence in that part which made it easy to detect the deflection of the alpha particles.
3