Balance the given chemical equation:
Al(s) + CuCl2(aq) → AlCl3(aq) + Cu(s)
Balanced equation: 2Al + 3CuCl2→ 2AlCl3 + 3Cu
Step 1: Write the unbalanced equation
Al + CuCl2→ AlCl3 + Cu
Step 2: Compare the number of atoms of reactants with the number of atoms of products.
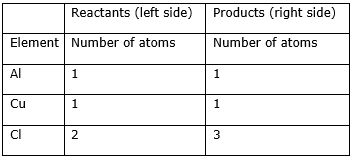
Step 3: Now, first we consider the element having unequal no. of atoms on both sides. Thus, first let us consider the chlorine atom. If we multiply 3 in the reactant (in CuCl2) and 2 in the product (AlCl3), we will get the equal number of atoms in both sides.
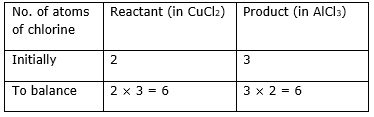
Step 4: Write the resulting equation:
Al + 3CuCl2→ 2AlCl3 + Cu
Step 5: Now check whether the equation is balanced or not by comparing the atoms
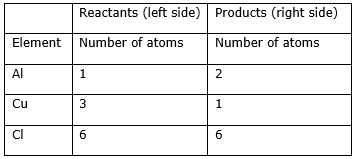
We find that the equation is not balanced yet. As the number of Cu and Al atoms are unequal on the two sides. First balance the Al atom.
Step 6: If we multiply 2 in the reactant (in Al), we will get the equal number of Al atoms on both sides.

Step 7: Write the resulting equation:
2Al + 3CuCl2→ 2AlCl3 + Cu
Step 8: Now check whether the equation is balanced or not by comparing the atoms.
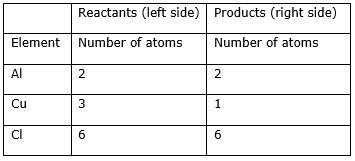
Step 9: Write the resulting equation:
2Al + 3CuCl2→ 2AlCl3 + Cu
We find that the equation is not balanced yet. As the number of copper atoms is unequal on the two sides.
Step 10: If we multiply 3 in the product (in Cu), we will get the equal number of atoms as in reactant (in CuCl2)

Step 11: Write the resulting equation:
2Al + 3CuCl2→ 2AlCl3 + 3Cu
We find that the equation is balanced now.
Step 12: Write down the final balanced equation:
2Al + 3CuCl2→ 2AlCl3 + 3Cu