Give two examples each of:
Redox reactions
The reactions in which oxidation and reduction are taking place at the same time are called redox reactions.
For examples:
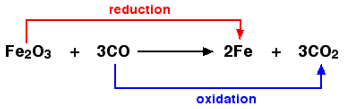
In the above example, Fe2O3 is reduced to Fe by losing oxygen atoms.
Thus, Fe undergoes reduction.
CO is oxidized to CO2 by gaining oxygen atom. Thus, C undergoes oxidation.
CuO + H2→ Cu + H2O
In the given reaction, CuO is reduced to Cu by losing oxygen. Thus, Cu undergoes reduction.
H2 is oxidized to H2O by gaining oxygen atom. Thus, H2 undergoes oxidation.
21