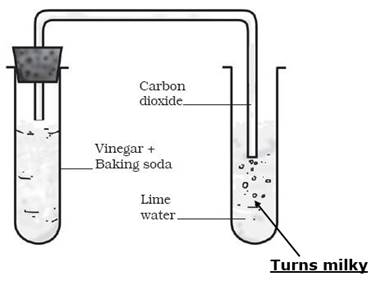
Give reason why:
Lime water turns milky when CO3 is passed through it?
When limewater (CaOH)2 is passed through CO2, the following reaction takes place:
(CaOH)2 + CO2→ CaCO3 + H2O
Limewater Calcium
Carbonate
i. In this reaction, when limewater comes in contact with the gas released in the form of an effervescence, it turns milky. This
chemical test for carbon dioxide gas.
ii. When limewater turns milky, it is confirmed that the effervescence is of carbon dioxide.
23