Show the formation of:
(a) MgO
(b) Na2O by the transfer of electrons.
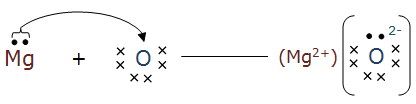
(a) Magnesium loses two electrons to attain a noble gas configuration, whereas oxygen gains two electrons to completely fill the valence shell.
Mg → Mg2+ + 2e‑
O + 2e-→ O2-
(b) Sodium loses one electron to have a completely filled valence shell, whereas oxygen gains two electrons to complete the valence shell.
Na → Na+ + e‑
O + 2e-→ O2-
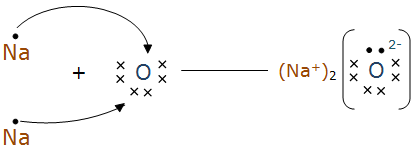
16