How are the following metals reduced:
(a) Metals which are highly reactive?
(b) Metals which are in the middle of the reactivity series?
(a) Highly reactive metals (e.g., sodium, potassium) are reduced from their compounds by electrolytic reduction. By electrolyzing the chlorides or oxides of these metals, the pure metal is deposited at the cathode.
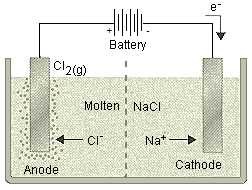
Electrolysis of molten sodium chloride
(b) Metals which come in the middle of the reactivity series are reduced from their compound using any of the following techniques.
i. If the compound is a metal oxide, then it is reduced to the corresponding metals by reduction using carbon (coke) in the presence of heat.
ii. If the compound is a metal carbonate, then it is converted into corresponding metal oxide by strongly heating it in the limited air (calcination). Later, metal oxides are reduced to metals using carbon.
iii. If the compound is a metal sulphide, then it is converted into corresponding metal oxide by strongly heating it in excess air (roasting). Then the metal oxide obtained is reduced to metal using carbon.