(a) Make a flowchart to explain the various steps involved in the extraction of metals from ores.
(b) Give four characteristics of ionic compounds.
OR
Give reasons only:
(a) Na, K and Li are stored under oil.
(b) Pt, Au and Ag are used to make jewellery.
(c) Hydrogen gas is not evolved when metals react with nitric acid.
(d) Aluminium is a reactive metal, yet it is used to make utensils for cooking.
(e) Aqueous copper sulphate solution should not be stored in an iron container.
(a)
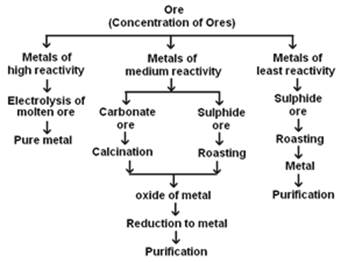
Flowchart: Extraction of metals from ores
(b) The characteristics of ionic compounds are,
i. Ionic compounds are hard solids. They are brittle in nature.
ii. They have high melting and boiling point.
iii. They are generally soluble in water, but they do not dissolve in solvents like kerosene, petrol etc.
iv. Ionic compounds conduct electricity in their molten/dissolved form. They do not conduct electricity in their solid form.
OR
(a) Na, K and Li react vigorously with air and water. Hence they cannot be kept in open or in water. They do not react with oil or kerosene. They are stored under oil to avoid contact with air and water.
(b) Pt, Au and Ag are lustrous. They shine brightly when light falls on them. Besides, these metals are easily malleable and ductile to make chains, rings, and so on. Thus they are used in jewellery.
(c) Nitric acid is a strong oxidizing agent. The hydrogen gas obtained from the reaction is quickly oxidized to water (H2O).
(d) Aluminium is a light metal. It is a good conductor of heat. It has a relatively high melting point. Due to the reactivity of aluminium, it combines with oxygen to form a layer of aluminium oxide over the surface of aluminium. This oxide layer prevents it from further reactivity of the metal. All these reasons make aluminium useful in making cooking utensils.
(e) Iron is more reactive than copper. So iron would displace copper from its salt solutions. In this case, the following reaction will occur:
Fe + CuSO4→ FeSO4 + Cu
Hence, there will no longer be copper sulphate solution in the container. It is advised to store chemicals with containers made of less reactive metals.