What is meant by saponification? Explain the process and its practical utility.
Saponification is considered the reverse reaction of esterification. Esterification is the process of producing esters by the reaction of an alcohol and a carboxylic acid. Saponification is the process in which an ester upon hydrolysis in the presence of a base gives back the alcohol and the carboxylic acid.
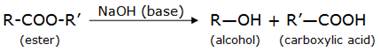
This process is called saponification because this is used in the preparation of soap. Soaps are sodium or potassium salts of long chain carboxylic acids.
11