What similarity do you observe in:
(i) Elements belonging to the same period.
(ii) Elements belonging to the same group.
Elements belonging to the same period have the same number of shells.
Example: Every element in the first period (top row) has one orbital for its electrons. All the elements in the second period (the second row) have two orbitals for their electrons.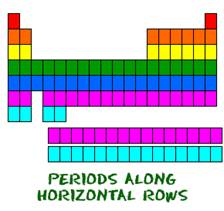
(ii) Elements belonging to the same group have same number of valence electrons.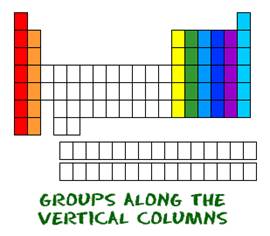
11