The position of three elements A, B and C in the Periodic table is given above. Giving a reason, explain the following:
(i) Element A is a non-metal.
(ii) An atom of element C has a larger size than an atom of element A.
(iii) Element B has a valency of 1.
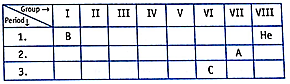
(i) Element A is closer to noble gas and is on the right side of the period and in the periodic table, non-metals are on the right side.
(ii) As we go down in periodic table atomic size increases, so the size of element C is greater than element A.
(iii) Element B is in the first group and it consists of elements having 1 electron in the outermost shell so it has valency 1.
25