Answer the following:
(i) What happens when a concentrated solution of sodium chloride is electrolysed? Write the equation of the reaction involved.
(ii) Why is the electrolysis of a concentrated solution of sodium chloride known as chlor-alkali process?
(iii) Name three products of the chlor-alkali process. State two uses of each product.
(i) When a concentrated solution of sodium chloride is electrolyzed, it forms sodium hydroxide (NaOH), chlorine gas and hydrogen gas.
Chlorine gas is formed at anode (positively charged) and hydrogen gas at cathode (negatively charged)
The reaction takes place is given as:
2NaCl + 2H2O → 2NaOH + Cl2 + H2
The above reaction is called chlor-alkali process.
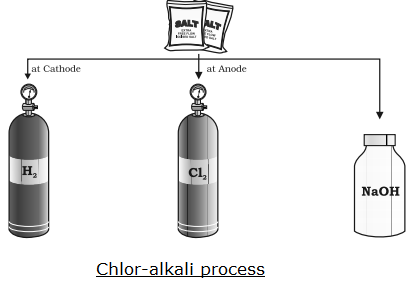
(ii) The electrolysis of a concentrated solution of sodium chloride known as chlor-alkali process because of the products formed:
a. The products formed in this reaction are NaOH (a strong base/alkali), chlorine and hydrogen gas.
b. “Chlor” means chlorine and “alkali” means base i.e. NaOH.
(iii) The three products of thee chlor-alkali process are:
Sodium hydroxide (NaOH)
Uses:
a. It is mostly used in soaps and detergents.
b. It is also used to make papers and artificial fibers.
Chlorine gas
Uses:
a. It is mostly used in medicines and in fuels.
b. It is used with ammonia for fertilizers.
Hydrogen gas
Uses:
a. It is used in treatment of water in swimming pools.
b. It is used as disinfectants and pesticides.
Note: Water of crystallization is the fixed number of water molecules present in one formula unit of a salt. Five water molecules are present in one formal unit of copper sulphate, i.e., CuSO4.5H2O