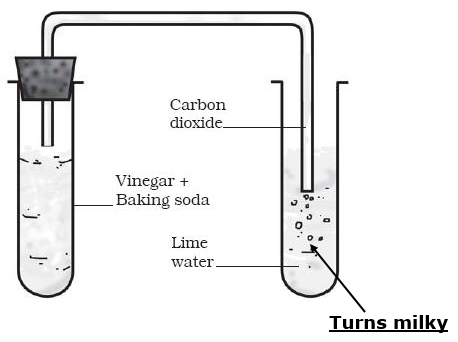
Acetic acid was added to a solid X kept in a test tube. A colourless and odourless gas was evolved. The gas was passed through lime water which turned milky. It was concluded that.
When any acid reacts with metal hydrogencarbonate, the products formed are salt, carbon dioxide (CO2) and water.
Metal hydrogencarbonate + Acid → Salt + Carbon dioxide + Water
Hence, when acetic acid reacts with sodium bicarbonate (NaHCO3), a colourless and odourless of carbon dioxide gas is evolved.
When carbon dioxide gas is passed through lime water [(CaOH)2], the following reaction takes place:
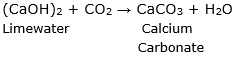
i. In this reaction, when limewater comes in contact with the gas
released in the form of an effervescence, it turns milky. This is a
chemical test for carbon dioxide gas.
ii. When limewater turns milky, it is confirmed that the
effervescence is of carbon dioxide.