Consider the following reaction:
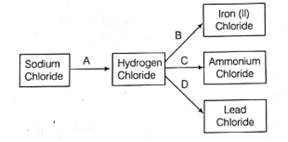
Here, A, B, C and D respectively are:
Step 1: When sodium chloride reacts with conc. H2SO4, it forms hydrogen chloride (HCl) and sodium sulphate (Na2SO4).
The reaction that takes place is:
2NaCl + conc. H2SO4→ 2HCl + Na2SO4
Thus, A is concentrated H2SO4
Step 2: When hydrogen chloride reacts with iron metal (Fe), it forms iron (II) chloride (FeCl2) and hydrogen gas (H2).
The reaction that takes place is:
Fe + HCl → FeCl2 + H2
Thus, B is Fe.
Step 3: When hydrogen chloride reacts with ammonia (NH3), it forms ammonium chloride (NH4Cl).
The reaction that takes place is:
NH3 + HCl → NH4Cl
Thus, C is ammonia (NH3).
Step 4: When hydrogen chloride reacts with lead nitrate [Pb(NO3)2], it forms lead chloride (PbCl2) and nitric acid (HNO3) .
The reaction that takes place is:
Pb(NO3)2 + HCl → PbCl2 + HNO3
Thus, D is lead nitrate [Pb(NO3)2]