The pH of a solution is 5.0. Its hydrogen ion concentration is decreased by 100 times, the solution will be:
The pH of a solution is 5.0 and if its hydrogen ion concentration is decreased by 100 times, the solution will be neutral because the decrease in hydrogen ion concentration represents an increase in the pH value.
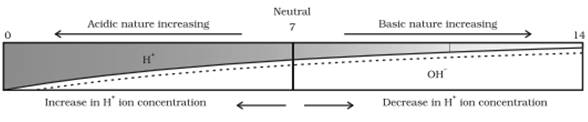
Hence, if the hydrogen ion concentration is decreased by 100 times the solution will change its medium from acidic to neutral (5.0 to 7.0),
10