In each test tubes A, B, C and D, 2mL of solution of Al2(SO4), in water was filled. Clean pieces of zinc were placed in test tube A, clean iron nail was put in test tube B, silver (Ag) was placed in test tube C and a clean copper wire was placed in test tube D.
Which of the following option (s) is/are correct about above experiment?
Zinc, Copper as well as silver are less reactive as compared to aluminium and hence cannot displace aluminium from its salt as they are placed below aluminium in activity series.
So the correct answer is d.
The activity series of metals is as follows:
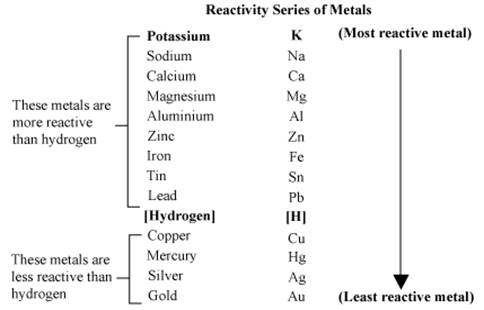
1