What are valence electrons? Does the number of valence electrons increase or decrees on moving from left to right in a period. How does valency of elements vary in the period?
● The electrons present in the outermost shell of an electron are known as valence electrons.
● The number of valence electrons increases on moving from left to right in a period. This is because on moving from left to right the electrons are added in the same shell.
● The valency of elements first increases in the period and then decreases. For example the trend of valency can be illustrated as follows:
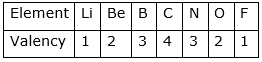
20