Four elements P, Q, R, and S have atomic numbers 12, 13, 14 and 15 respectively. Answer the following questions giving reasons.
(i) What is the valency of Q?
(ii) Classify these elements as metals and non-metals.
(iii) Which of these elements will form the most basic oxide?
(i) The electronic configuration of Q is :2,8,3. It has three valence electrons, so it has a valency of three.
(ii) We can classify an element as a metal or non-metal by looking at its position in periodic table. Those lying on left side of periodic table are metals and those lying on right side are non-metals.
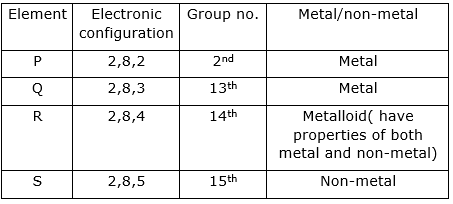
(iii) The element P is most metallic because it can easily loose two electrons and lies on the left side of the periodic table. Metal oxides are basic in nature, therefore oxide of P will be most basic.