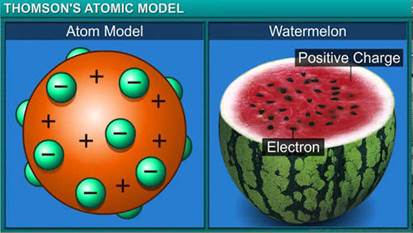
(a) Why is Thomson’s model of an atom compared with watermelon?
(b) Why do isotopes have different mass numbers?
a. According to Thomson’s model of the atom, the atom is a sphere of positive charge with negatively charged electrons embedded in it. It is compared with a watermelon because the sphere of positive charge represents the red, edible part of watermelon whereas electrons embedded in a positively charged sphere represent the black seeds.
b. Isotopes of an element differ in the number of neutrons in the nuclei so they have a different mass number as the mass number is the sum of a number of protons and neutrons in the nucleus.
6