Write the two examples of the crystalline form of carbon.
In the crystalline form of carbon, many atoms are bonded together in a repeating pattern. The arrangement of atoms in the crystal differs for each form of carbon. Two forms are:
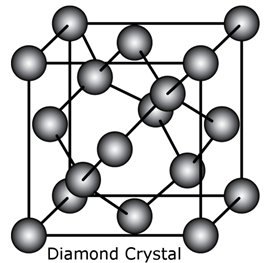
i. Diamond.
Each carbon atom is covalently bonded to four other carbon atoms. The arrangement of carbon is shown below:
ii. Fullerene:
each carbon atom is covalently bonded to four other carbon atoms. Each sphere contains 60 carbon atoms, and each carbon atom is bonded to three others by single covalent bonds.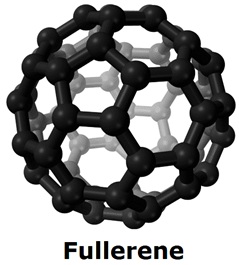
1