Why is graphite smooth?
In graphite, each carbon atom is bonded to 3 other carbon atoms in the same plane giving rise to a hexagonal array. The structure of graphite is formed by stacking these hexagonal layers over one another and these layers have weak inter-molecular forces.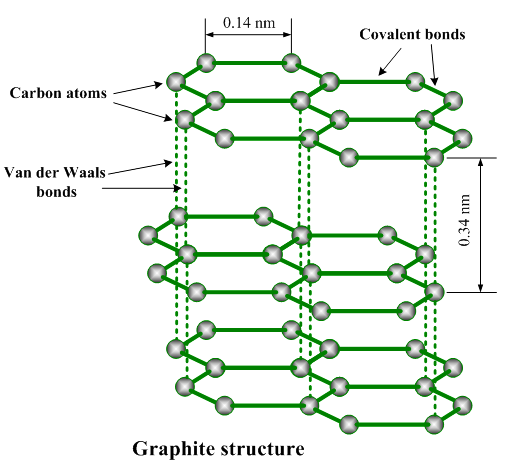
This structure results in the smoothness of graphite.
3