Write the electron dot structure of C3H8.
C3H8 contains 3 carbon atoms and 8 hydrogen atoms.
Now, each carbon atom has 4 electron and each hydrogen atom has 1 electron each.
So, in total 3×4 + 1×8 = 12 + 8 = 20 electrons.
And, 1 single bond = 2 electrons.
∴ 10 single bonds.
There now plot the electrons on the atoms as shown, and then jin the single bonds.
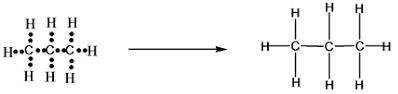
This is the final structure of propane i.e. C3H8
4