Give a detailed explanation on the following.
Properties of acetic acid
Acetic acid (CH3COOH) is a weak acid and belongs to the family of carboxylic acids.
Physical Properties:
Acetic acid exists in liquid state at room temperature. Pure acetic acid has a melting point of 290K and often freezes when kept in cold climates.
Chemical Properties:
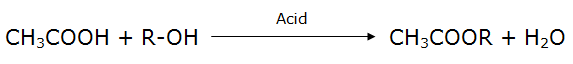
(a) Reaction with alcohol:
Acetic acids react with alcohols in the presence of an acidic medium to form esters. Esters are sweet smelling substances and are often used in perfumes and as flavouring agents.
(b) Reaction with base:
Like other acids, acetic acid reacts with base to form salt and water. The reaction is called neutralization reaction.
![]()
(c) Reaction with carbonates and bicarbonates:
Acetic acid reacts with carbonates and bicarbonates to form salt, carbon dioxide and water.
2CH3COOH + Na2CO3→ 2CH3COONa + CO2↑ + H2O
CH3COOH + NaHCO3→ CH3COONa + CO2↑ + H2O