Give account of chemical properties?
(i) combustion of ethanol
(ii) oxidation of ethanol
(iii) reaction of C2H5OH with sodium metal
(iv) dehydration of C2H5OH
(v) reaction of C2H5OH with ethanoic acid
(i) Ethanol upon combustion gives carbon dioxide and water.
C2H5OH + 3O2→ 2CO2 + 3H2O
(ii) Ethanol is oxidized to ethanoic acid when treated with oxidizing agents like acidified potassium dichromate
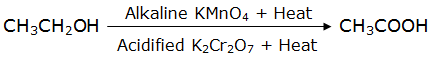
(iii) Ethanol reacts with sodium metal to liberate hydrogen gas.
2C2H5OH + 2Na → 2C2H5O-Na+ + H2
(iv) Ethanol when dehydrated using conc. H2SO4 forms ethene.
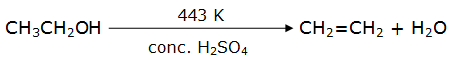
(v) Ethanol (C2H5OH) reacts with ethanoic acid (CH3COOH) in the presence of acidic medium to produce ethyl ethanoate which is an ester.
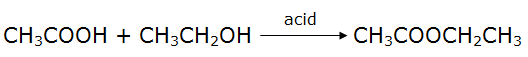
40