An organic compound A with molecular form C4H8O2 on alkaline hydrolysis gives two compound B and C. C on acidification with dil. HCl gives D. Oxidation of B with K2Cr2O7/H2SO4 also gives D. Identify A, B, C and D and explain all the reactions involved.
A – ethyl ethanoate
B – ethanol
C – sodium ethanoate
D – ethanoic acid
Esters upon alkaline hydrolysis gives back the carboxylic acid and the alcohol. The acid is initially obtained in its salt form. The reaction is called saponification.
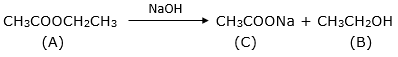
The salt (sodium ethanoate) is treated with dil HCl to obtain the carboxylic acid.
![]()
Ethanol is oxidized to ethanoic acid in the presence of acidified potassium dichromate. Here, the acid used for acidification is H2SO4.
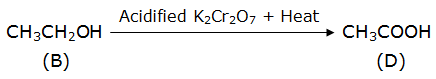
41