The diagram shows the molecule, ethyl propanoate.
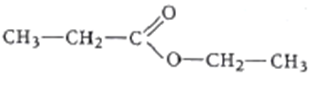
How many bonding pairs of electrons are there in the molecule?
Number of C-H bonds = 10 = 10 bond pairs (bp) of electrons
Number of C-C bonds = 3 = 3 bp of electrons
Number of C-O bonds = 2 = 2 bp of electrons
Number of C=O bonds = 1 = 2 bp of electrons
Total number of bp = 10+3+2+2 = 17
5