Identify a deadly poisonous gas X found at the higher levels of atmosphere. Write its chemical formula and equation of its formation. Why is damage to X layer a cause of concern?
Ozone is a simple chemical compound that contains only oxygen atoms, and its effects depend on where in the atmosphere it occurs. In the upper stratosphere, it forms a protective shield against solar ultraviolet radiation, but near the ground, it's a pollutant that can cause respiratory ailments in humans and animals. The creation and destruction of stratospheric ozone depend primarily on natural processes, but near the ground, industrial processes are mostly responsible for its creation.
Hence ozone is the gas found at higher level of atmosphere (stratosphere).
Chemical Composition
An ozone molecule consists of three oxygen atoms (O3), whereas the stable form of oxygen that normally exists in the atmosphere consists of only two. When certain chemical processes make an extra oxygen atom available, the highly reactive atom binds readily with an oxygen molecule.
Then these UV rays split molecular oxygen (O2) into free oxygen (O). Afterwards, this free oxygen reacts with molecular oxygen; it forms ozone (O3).
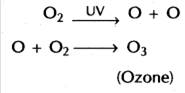
Damage to this layer is of great concern as this ozone protects the earth from harmful UV radiations of sun, by allowing only some percent of rays to fall on the earth’s surface. Ozone acts as a filter of sun rays. These rays can cause skin cancer in human and can also affect other living organisms.