Draw a labelled diagram to show the sublimation of Ammonium chloride.
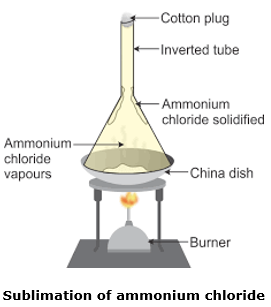
Ammonium chloride (NH4Cl) is taken on a china dish. It is covered by placing an inverted funnel. The neck of the funnel is plugged using cotton. This is heated with the help of a burner as shown in the figure.
One can see that ammonium chloride starts to sublime (to convert into gaseous phase without entering liquid phase). The ammonium chloride vapours try to escape, but the funnel is completely sealed. Therefore, the ammonium chloride vapours after rising up lose energy and get solidified along the walls of the funnel. This process from vapour to solid state is also called sublimation.
19